Troikaa Group (Troikaa Pharmaceuticals Limited. and Troikaa Pharmachem Private. Limited.) (“Company” or “us” or “we“) respects your privacy and is committed to protecting your personal data.
This notice and request for consent (“Notice“) will inform you about how the Company proposes to collect, handle, store, use, disclose and transfer (“Process“) your personal data.
Note: We may collect your personal data directly from you, from third party’s companies, regulators or governmental authorities who may have your personal data, or from publicly accessible sources such as your social accounts where you have made your personal data publicly available.
Note: We may undertake the abovementioned activities either ourselves or through third parties such as vendors, service providers, other regulated entities such.
Contact Information:
For more details about how we Process your personal data for various purposes, your rights under the law, and our privacy practices, please read our Privacy Policy.
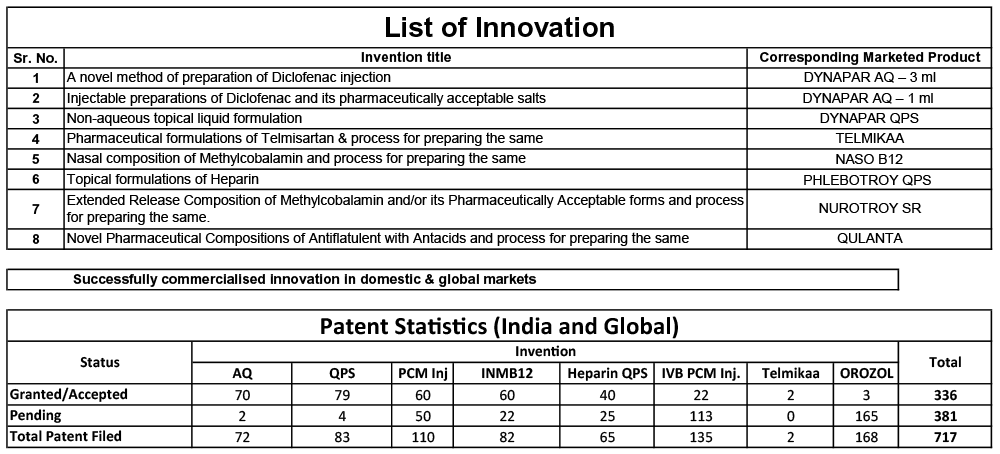
Our R&D Department is approved by the Department of Scientific & Industrial Research, Government of India and has an innovation-driven approach. This approach has helped us in creating path-breaking brands.
Each patentedbrand at Troikaa has stemmed from original innovations;of which the lead time from concept to commercialization has been effectively maintained from 6 to 10 years. It may affect our short-term profitability but it drives long-term growth.Our R&D innovations have helped steer us into global markets and made us a well-recognized company worldwide.
Many of Troikaa’s products like Parenteral Emulsions (Propofol; Etomidate injection), inhalation agents for anaesthesia (Isoflurane, Sevoflurane), Sublingual Tablets, etc. have been created after extensive research, dedicated expert manpower, thorough and stringent clinical trials. This has helped create a niche in the industry,which has spearheaded our rapid sales.
